electron dot structure of butane|C4H10 Lewis Structure : Tuguegarao Hul 26, 2013 — 73K views 11 years ago. A step-by-step explanation of how to draw the C4H10 Lewis Dot Structure (Butane). For the C4H10 structure use the periodic table to find the total number of . Die besten Online Casinos für Spielautomaten 2024 . Als Casino Experten zocken die Casino.ch Redakteure selbst regelmässig Automatenspiele. Unsere Top Liste stellt eine Auswahl empfehlenswerter Online Casinos mit Spielautomaten für Schweizer Spieler dar. Sowohl Spielauswahl als auch Bedingungen konnten uns überzeugen.
PH0 · Lewis Structure of C4H10 (Butane) (In 6 Simple Steps)
PH1 · Draw electron dot structure of butane.
PH2 · Draw electron dot structure of Butane.
PH3 · C4H10 Lewis Structure: How to Draw the Lewis Structure for C4H10
PH4 · C4H10 Lewis Structure
PH5 · C4H10 (Butane) Lewis Structure in 6 Steps (With Images)
PH6 · C4H10 (Butane) Lewis Structure in 6 Steps (With
PH7 · Butane C4H10 Lewis Dot Structure
PH8 · Butane
PH9 · 4.5: Electron Dot Structures of Organic Compounds
SIAS Online 3 - St. Paul University Philippines. Log in to access your student information, academic records, and online services.About Time Difference Calculator. By using the Time Duration Calculator, one can easily find the actual time difference between two specific points in time (the starting time point and the end time point).In order to use this calculator, you should enter the values of both specific time points in hours, minutes, and seconds.The result will be displayed in .
electron dot structure of butane*******Lewis electron dot structure of butane (C 4 H 10) : With the help of electronic configuration, it is clear that carbon has four and hydrogen has one valence electron. C = 1 s 2 , 2 s 2 , 2 p 2Hul 26, 2013 — 73K views 11 years ago. A step-by-step explanation of how to draw the C4H10 Lewis Dot Structure (Butane). For the C4H10 structure use the periodic table to find the total number of .
Nob 17, 2022 — Figure 4-26 (left): Adapting the electron dot structure of butane to make butan-1-ol. One of the hydrogen atoms on the leftmost carbon is replaced by a hydroxyl (OH) fragment with seven valence .
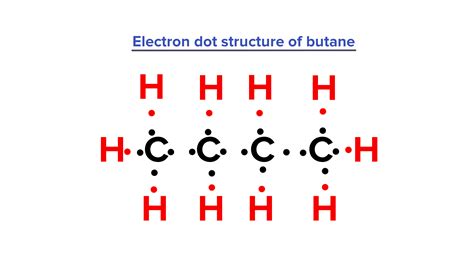
May 15, 2012 — A video explanation of how to draw the Lewis Dot Structure for Butane, along with information about the compound including Formal Charges, Polarity, Hybrid O.4 days ago — Electron dot structure of butane is given as: We can see that there are four valence electrons of carbon and one valence electron of hydrogen because the .
Butane. Formula: C 4 H 10. Molecular weight: 58.1222. IUPAC Standard InChI:InChI=1S/C4H10/c1-3-4-2/h3-4H2,1-2H3 Copy. IUPAC Standard .Formula: C 4 H 10. Molecular weight: 58.1222. IUPAC Standard InChI: InChI=1S/C4H10/c1-3-4-2/h3-4H2,1-2H3. IUPAC Standard InChIKey: IJDNQMDRQITEOD-UHFFFAOYSA-N. CAS Registry Number: 106-97 .
Hun 22, 2023 — C4H10 (Butane) lewis structure has a single bond between the Carbon-Carbon atoms (C) as well as between the Carbon atom (C) and Hydrogen atom (H). The four Carbon atoms (C) are at the center and .
This is the C4H10 Lewis structure: Butane. For Butane, we have a total of 26 valence electrons. Whenever we see the ending, "ane", we know that we're going to have .4 days ago — Hint: Electron dot structure is also known as Lewis dot structure. Lewis dot structure can be made of covalent compounds. To draw the electron dot structure of any compound, we should know its formula. In this type of structure, electrons are denoted with dots and only valence electrons (electrons in the outermost shell of the atom) are .Even though diamond and graphite are made up of carbon atoms only,the molecular structure of diamond is different form that graphite. Diamond has 3D structure of graphite consists of layer of carbon staked above one another.
Ene 6, 2023 — The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule. They are similar to electron dot diagrams in that the valence electrons in lone pairs are represented as dots, but they also contain lines to represent shared pairs in a chemical bond (single, double, triple, etc.).Set 23, 2023 — Butane is a saturated hydrocarbon and belongs to the Alkane series. In this article, we will discuss the electron dot structure of butane. Electron dot structure of C4H10. We will discuss the electron dot structure of butane through the following points . 1. Formula of butane. 2. Electronic configuration of carbon. 3. Electronic configuration .Okt 29, 2021 — Step 4) Count the electrons in each structure. Each of these structures has 24 electrons. 3 lines = 6 bonding electrons. 18 dots = 18 nonbonding electron. total = 24 electrons . Do these match the counts you got in step 1? BF 3: should have 24 electrons (from step 1) Our structure has 24 electrons – MATCHES. PF 3: should have 26 .May 14, 2021 — A step-by-step explanation of how to draw the C5H12 Lewis Dot Structure (Pentane). We'll also draw the skeletal structure and molecular structure for Pentan.
Ene 25, 2023 — Hence, Butane is non-polar. Butane Lewis Structure. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Each dot represents an electron, and a pair of dots between chemical symbols for atoms represents a bond.To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. . (not shown) are implied to have single bonds to Carbon. You can view a better structural formula of butane at en .A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.Peb 13, 2019 — Introduction. Observe the following drawings of the structure of Retinol, the most common form of vitamin A. The first drawing follows the straight-line (a.k.a. Kekulé) structure which is helpful when you want to look at every single atom; however, showing all of the hydrogen atoms makes it difficult to compare the overall structure with other .Butane is a four-carbon alkane, hence its chemical formula is C 4 H 10. Butane has two structural (also known as constitutional) isomers: normal butane, also known as unbranched butane, and isobutane, commonly .Butane is a colorless gas with a faint petroleum-like odor. For transportation it may be stenched. It is shipped as a liquefied gas under its vapor pressure.To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. . (not shown) are implied to have single bonds to Carbon. You can view a better structural formula of butane at .electron dot structure of butane C4H10 Lewis Structure Hun 22, 2023 — C4H10 (Butane) lewis structure has a single bond between the Carbon-Carbon atoms (C) as well as between the Carbon atom (C) and Hydrogen atom (H). . Formal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons . In the above lewis dot structure of C4H10, you can also represent each bonding electron .The resulting Lewis electron dot structure displays a triple bond connecting a carbon and an oxygen atom, each holding a lone pair of electrons. Solved Examples. Problem-1: In terms of electron dot formulas, define the electron structure of the carbonate ion CO 3 2-. Solution: One potential electron dot formula for the carbonate ion is
Drawing C is a Lewis electron dot structure for methane. Figure 1 Alternative Representations of Methane. Lewis Electron Dot Structures. . Butane is a linear molecule; you can trace a path from the first to the fourth carbon atom without lifting your pencil from the paper.

Draw the Lewis structure of NOF. Draw nonbonding electrons using dot notation and bonding electrons as a bond. Draw and explain the Lewis structure for CCl4. Draw the Lewis dot structure for acetamide, CH3CONH2, and determine the formal charge of each atom of this molecule. Write the Lewis structure for C_2H_2.electron dot structure of butane(ii) The electron-dot structure of M g O is: Magnesium donates its two electrons to the oxygen atom to form an ionic bond in magnesium oxide, M g O (iii) The electron-dot structure of H 2 O is: The oxygen atom shares its one electron with each hydrogen atom to form a covalent bond in the water molecule. (iv) The electron-dot structure of H C I is:
Viral Solid na kantotan ngayong 2024 - Sarappinay provides the latest pinay sex videos and pinay sex scandals. Watch the latest kantutan videos online here. Home; Categories; Popular; Free Facebook Leaks; Contact; Search. Viral Solid na kantotan ngayong 2024. Categories / Tags Scandal. Related Videos. 2:39.
electron dot structure of butane|C4H10 Lewis Structure